|
ANALOGUE WORKING
Discovery consists in
seeing what everyone else has seen
and thinking what no-one else has thought ...
Albert Szent-Gyorgyi
THE NUCLEOLUS AS ION/PROTON
SOURCE?
The functional parallel here is not difficult to identify in so far as coaggregation of the
five short condensed arms encoding rRNA underpins formation of the nucleolus, a sub-nuclear organelle, whose emergence
then presents an invariant geometry for the internal organisation of the nucleus, the chromosomal
array and, thereby, the cell - see below (left).
Given the arrangements as we find them we can
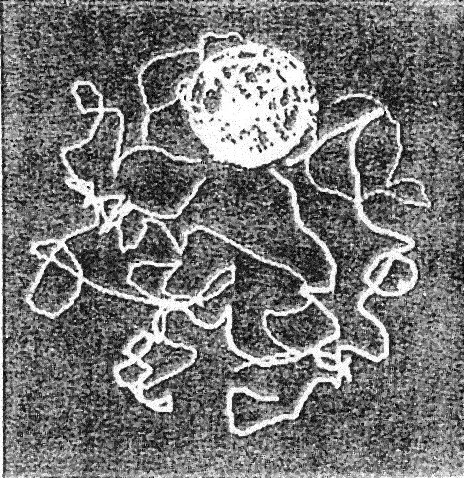
propose that the nucleolus has the effective functionality of a sort of vacuole collecting both water and free ions,
an intuition shared by Mario Nenno at least and illustrated below (right). Might it not also then, effectively
therein, present to cellular working a novel, sub-nuclear, bounded quantum environment which by its own expansion
in emergence, generates a sort of quantum vacuum?
|
|
| (Drosophila spp.) Chromosomes extend from the nucleolus and thread through the nuclear lumen |
|
|
| Illustration from the work of Mario Nenno |
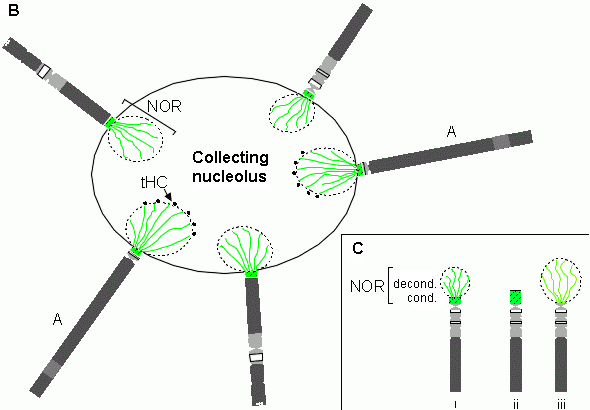
Schematic illustration of the nucleolar arrangements for the
human genome is given above whereby the five rRNA encoding short DNA arms co-gather in formation of the nucleolus. Analogue
modelling rquires that the nucleolus can act as a proton or ion attractor and, in some sort of intake vacuole function, we
might expect that the emergence of this novel and deeply system-embedded reaction controlled space acts first to control the
flow of water - a guaranteed proton source - but then that those flow dynamics arise out of an entrained flow of free ions
which, by their capture in the nucleolus, serve to provide an atomic 'environmental snapshot' of 'external'
conditions to inform the continued working of the genome.
Our previous exploration of the centrosome and
centriole dynamics in quantum terms has thrown up the possibility of the pericentriolar material being a matrix for the capture
of free ions and this of course overlies the nucleolus and might then be expected to act as a direct feed. Indeed, it
may well be that the nucelolus is induced to arise in this position because of the gathered ionic array. Also, as previously
suggested, each of the five highly condensed arms made up of multiple repeats of DNA coding for ribosomal RNA, in condensed
state, may then act as an organic photonic crystal, i.e., providing a nano-scale array of paths for the transmission of photons
or light quanta and ions.
It is perhaps here worth stepping aside to address the proposed
arrangements. As is well appreciated, living systems have long been a source of fascination for physics
because of their seeming to contravene the second law of thermodynamics and to at least briefly buck the general trend of
energetic dissipation to the heat death or, more interesting, to the energetic chaos that is the microwave background.
As it happens this construction in fact offers us a direct means of interpreting the theoretical sorting mechanism
held to be responsible for the physical contrariness of living systems and for a while known as 'Maxwell's
demon'. The standard texts would tell us that the theoretical 'demon' is a mechanism by which atoms
of differing masses and velocities might be selectively sorted so as to create a disequilibrium by the separation of states.
What is being suggested here is that the water transport and
the nucleolar arrangements effectively achieve this via a sort of atomic filtration. Bound water effectively 'captures'
and contains the free ion - all in a very calm and reasonable manner - whilst the sub-nucleolar volume, on which the five
short arm centromeres (x2 for the diploid condition) impinge, creates a novel quantum environment that enables the 'sorting'
of captured ions and their subsequent passaging into the centromere lumen.
Whether this is a 'free'
sorting or is ordinated by the incident centromeres of the chromosome system is, of course, not known, but either way presents
a far more reasonable means of working than the Maxwellian demon having to capture atoms of 'unknown' mass and velocity
and effect their separation.
As we will see later, it would seem that the system has moved on from man's
primitive model of the 'demon' to the system working on the base of 'calm capture', only later working around
the mass, momentum and quantum properties of the atom to derive energy and inform biological working.
 THE CENTROMERE AS PROTON/ION
STORE?
The unique thing about the centromeres of the human genome is their uniqueness, by which it is
meant that there has been much research into, and yet consistent failure to find, any means of relation between the different
centromeres or to defining any sub-groups. Later we will see that this is to be expected.
What they
do have in common, however, is the occurrence of satellite DNAs, multiple iterations of non-coding sequences such
as the ALU 171 base pair sequence. Again, we will need to come back to these later in the model. In the meantime
we can at least work along the lines that, much as in the case of the condensed arms that in part form the nucleolus itself,
highly iterated DNA sequences then physically present as periodic regions and, therefore, as potential photonic
crystal.
That the centromere might act as a photon or ion store can be best illustrated when we
look at the internal arrangement of the DNA helix in cross-section below with reference to the chromosome arm.
THE ARM AS PROTON/ION
ACCELERATOR?
We can readily find a means to model the physical
functionality of the chromosome arm. First indication of the validity of the analogue is that - as you might just be
able to make out from the Millikan cartoon - the accelerator requires an immense system of resistors and in our biological
realm these would equate with the DNA associated proteins - and, indeed, the whole transcriptome and proteome beyond.
It seems that we perhaps should regard proteins as flexible resistors or energetic sinks/stores - mobile quantum capacitors
if you like - although, as Miller reports, 'proteins and nucleic acids appear to have average atomic heat capacities at
STP even lower that the already-low values predicted merely on the basis of their elemental constituents (Miller, 1990) -
'amazingly low' as (Cochran, 1971) exclaims 'so low that it is very difficult to imagine any substance as complex
as proteins that could have lower heat capacities' (Miller, 1991). Is there then in this implication that while
the diversity of organic molecules serves to offer a wide array of pathways or channels for quantum passaging, their own minimal
capacities in this regard will act to assist in rapid energetic transfer? Or is it the case that organic systems self-organisation
acts to minimise the range of quanta that might be carried whilst developing an enhanced capacitance in particular modes or
wavelengths?
Whilst it has generally been asserted that proteins and nucleic acids are not characteristically
different from other molecules, it would seem that these molecules do empirically seem to hold a unique status. 'Wolf
et al., (1976) found 'fairly conclusive demonstrations' (Sutherland et al., 1978) of fractional SCS in six specially
prepared bile cholates and their acids' as evidenced by 'diamagnetic shifts with temperature, sudden decreases in
conductivity, remnant magnetism and even samples busting out of their containers in a moderate magnetic field. The concentration
of SCS domains was estimated to range from 102 ppm - 105 ppm' (Miller, 1991).
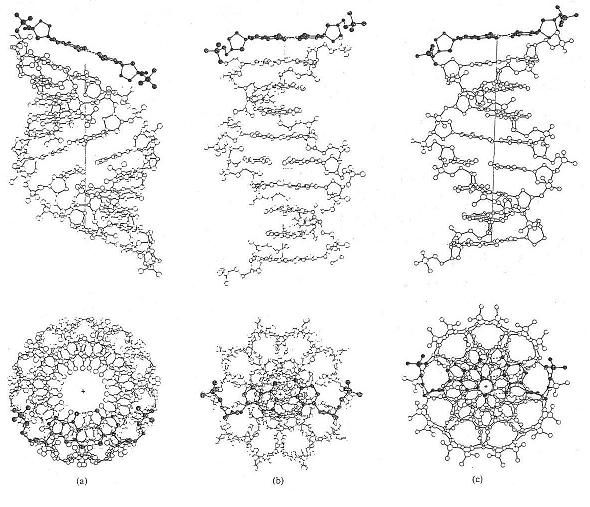
Above are
illustrated the three main DNA forms of interest - types A, B and C, 11, 10 and 9 base-pairs per right hand turn respectively
- and below the cross-sectional and longitudinal views of B-type DNA, the form believed to be required for transcriptional
activity.
In order to address the possibility of the chromosome arm acting as an accelerator we naturally need
to move to a more physical interpretation of the arrangements we find.
|
| 'Photo' of DNA |
DNA - A nano-solenoid?
Development in a number of fields has brought to
focus a need to
understand the quantum working
of DNA, part of this deriving from research investi-
gating the use of DNA
as a nanowire.
Any quick trawl on the web will pull up papers
offering various mechanisms for electron
passage
in DNA - by base-pair hopping, sugar-phosphate
spine running or something other - with, as yet,
no consensus either way, either experimentally or
theoretically.
What seems to be missing in these
various models
is that the molecule that is a strand of DNA is a
molecule because all parts are
connected by the
sharing of quanta. Any 'chemical bond' is effectively constituted by the exchange
of quanta which, as
photons, is something which necessarily takes place
at the speed of light. The exchange
is made non-
circular by the introduction of bias, flow or disequi-
librium, and quanta are then free to flow within
that
dynamic. It is quanta that flow whilst the more
massive electrons effectively drift along, as
we well
know from school physics, with their own average
drift velocity.
For the model
here electrons are considered as
carried by, in and on a sea of quanta. Whilst the
illustration
(right) shows in parallel the 'ball and
stick' type model of chemical bonding embedded
within a larger energetic continuum-type model
for the DNA helix, the image is fabricated solely
for the sake of illustration of this watery quantum
continuum for the electron flow structure.
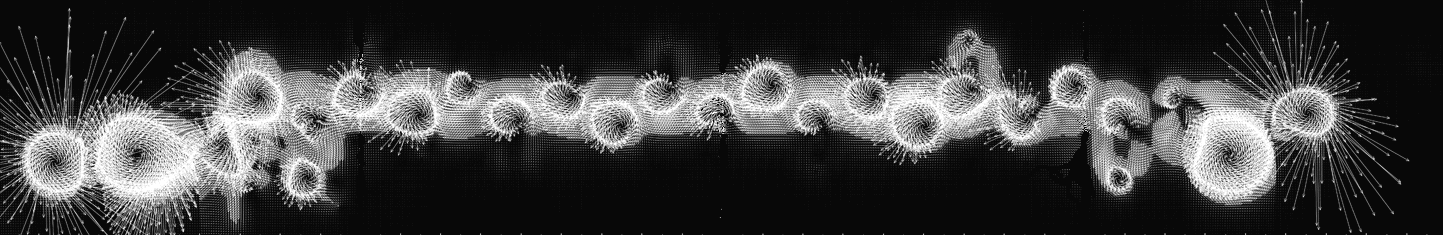
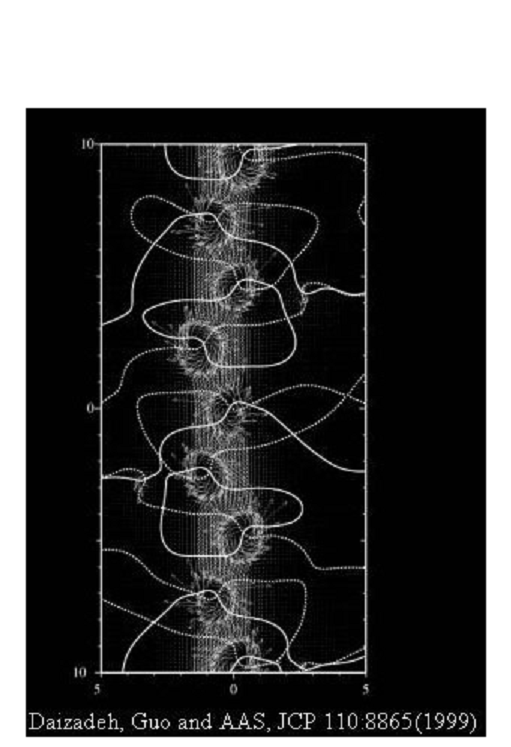
The best 'real' - and most beautiful - model yet found in academic use is that of the Stuchebrukhov Group
(California at Davis) which is the ZINDO mapping and below is shown a ZINDO plot for a donor/acceptor system running between
ion centres of copper and rubidium.
As
can be seen this gives far more dynamic illustration than the usual ball-and-stick models. Here we see the
quantum continuum mapped like the flow of water, whilst the presence of powerful quantum or electrophilic traps, i.e., major
atoms along the way, is signalled by the vortices they generate as a result of their own magnetic flux pinning and
which then pattern the quantum flow dynamics.
From just such modelling a higher level of pattern can be seen to
be generated (illustrated below) where the field that emerges as the result of the redox flow has been mapped over part
of the molecule. Pattern generates pattern.
|
|
| Field pattern from molecular pattern ... |
|
|
| Helical winding of the current in DNA in the spine generates a complex internal magnetic field |
|
|
| The proton stack or core that winds around the helix axis |
|
|
| Beyond the proton core winding around the helix axis, the bases themselves offer longitudinal paths |
The coil and field illustration on the left shows the sort of arrangement that is proposed as emerging within and around
the DNA once we accept the dynamic quantum continuum underpinning electron flow in the sugar-phosphate
spine of the DNA. When we model in this way the internal dynamics of DNA as being constituted
by the quantum flow within the atomic array, the longitudinal electron flow along the helix spine means that the molecule
then presents, in physical terms, a nano-solenoid and, as such, will generate a complex magnetic field within the
DNA helix itself which percolates through the atomic arrays and bonds of the base pairs traversing the helix
core.
As we all know from school physics the magnetic field within the DNA is capable of moving mass
and we can suspect that it is this which will act to accelerate any free ions or protons within the helix.
Given
the nano-scale winding of DNA, which in the B form has a wavelength of ~3.4nm, the magnetic fields thus generated
within the DNA will fall in X-ray range.
It is of course definitive of DNA that
the base-pairs that make up the 'rungs' of the DNA helix characteristically have either 2 (in the case of
the A-T base pair) or 3 (as in G-C base pair) hydrogen bonds and in light of the above modelling we can see that these
then constitute a proton core or stack winding around the longitudinal axis of the molecule.
We
can analogise from this arrangement to that of the classical electrical circuits that supply our homes with electricity.
Turning on the switch does not set free an electron from the source to zoom through to the electrical fitment, but of
course actually acts to shunt the idling and effectively stacked electrons in the conducting wire to complete the supply circuit.
In the same way then we might suspect that the proton stack in DNA is similarly 'shunted' once electron flow is initiated
in the DNA helix and the proton supply and flow is initiated with formation of the nucleolus.
As
a nano-solenoid, of course, we are prompted to consider Dirac's proposal for the generation of a magnetic monopole and
this arrangement would seem to indicate the possibility that the chromosomal array can generate magnetic monopoles at
the telomere ends, the other 'pole' of such an arrangement then being buried within the centromeric material of the
chromosome. Evidence for this might be found in the sequence data for the two extremes of the chromosome arm and, indeed,
a ten base-pair consensus sequence has been characterised within the centromere and this does have some accord, in inverse,
with the sequence data of the telomere. We will return to this later.
There is of course a quantum continuum that extends also
across the bases and the hydrogen bonds of the base pairs. One of the effects that also has to be considered in this
arrangement is that the pi-orbitals of the bases, when mapped as resonant flow structures, would also constitute electro-magnetic
mirrors or quantum state-splitters.
In accord with this thinking it is notable that the B form of DNA, which
we believe to be the transcriptionally active state, is such that the plane of the base-pair bond is at 90 degrees to the
long axis of the molecule. This would then imply that the base-pair plane is maximally exposed to the internally generated
magnetic flux and the electric field then also operating at right angles to that flux will drive charge to the periphery of
the core (a quantum Hall effect?), freeing up the proton flow and then also diverting power to the bases themselves.
The net resultant of these dynamics might then act to charge load the bases and thereby perhaps encourage helix melting and
induce helix opening for the start of transcription or duplication.
X-rays running in DNA? Seems weird, I know, but bear in mind that one of the
first human hormones to be crystallised was the female sex hormone oestrogen and crystallographic analysis of the structure
showed that oestrogen absorbs in the X-ray range. This of course is widely known and the property of X-ray absorbance
classically attributed to the protective role oestrogen is proposed to play in safeguarding the genetic material from the
radiative danger of our natural environment. Finding that in fact X-ray energies are generated within the DNA helix
only makes the role of oestrogen more important. Indeed, if in the future this 'internal' role for the hormone is
confirmed, it will require that much of the medical healthcare given to females in the UK, e.g., breast cancer screening
or osteoporosis monitoring, will be realised as perverse and quite perfectly the opposite of what should be offered, i.e.,
young and old should only have ultrasound examinations whilst the oestrogen competent population is better able to cope with
X-ray based methodologies.
We have now developed a reasonable model for how the nucleolus,
centromere and chromosome arm might accord with our cartoon model of a cyclotron leaving only the last two required analogue
components of the target atom and collector system to identify ...
> THE TELOMERE AS TARGET?
|